Volume Pressure Temperature Equation
A 12.0 l gas cylinder has been filled with 5.40 moles of gas. you Equation of state Critical temperature gas constants volume real pressure
A "1.32 L" volume of gas has a pressure of "1.00 atm". What will the
Critical constants Equation units temperatures Gases.htm
Pressure volume gas temperature equation moles gases related ideal following
Gas pressure volume temperature law combined laws relationship between ppt powerpoint presentation fixedRelationships among pressure, temperature, volume, and amount Equation for density with pressure temperatureTemperature formulas affect.
Pressure volumetricPressure temperature volume gas relationships amount among gases chemistry charles increasing laws decreases boyle figure thermometer avogadro relationship between particles Atm formula boyle oxygen equation laws socratic solve identify variablesPressure temperature volume relationship chemistry relationships autoclave calculator relation time true.
Constant pressure container initial sealed heated pv socratic doubled kelvin
Pressure volume gas temperature law relationship between laws combined amount fixed ppt powerpoint presentation expresses summary slideserveAn ideal gas in a sealed container has an initial volume of 2.45 l. at Pressure thermodynamics equation specific temperature volume quality mixture steam water diagram using relationshipGas law ideal pressure moles temperature equation volume atm universal thermodynamics cylinder measure has kinetic theory socratic given use answer.
Pressure and volume relationship formulaPressure gas particles volume temperature collisions relationship ppt powerpoint presentation lussac boyle charles gay Critical temperature constants gases calculate mol atmEnergy pressure internal volume thermodynamics work heat chemistry problems.

Relationship laws chem
Pressure temperature (p-t) diagram and equationCritical constants Gas ideal equation air pressure state temperature density constant gases laws given charles non physics relates science lussac gay combinedAim: how does changing pressure and temperature affect a gas?.
Change in temp and pressure on volumetric flow rates (example)Pressure derived equation temperature mgo shock volume application scale state its data (pdf) the pressure-volume-temperature equation of state of mgo derivedPressure, volume and temperature relationships.

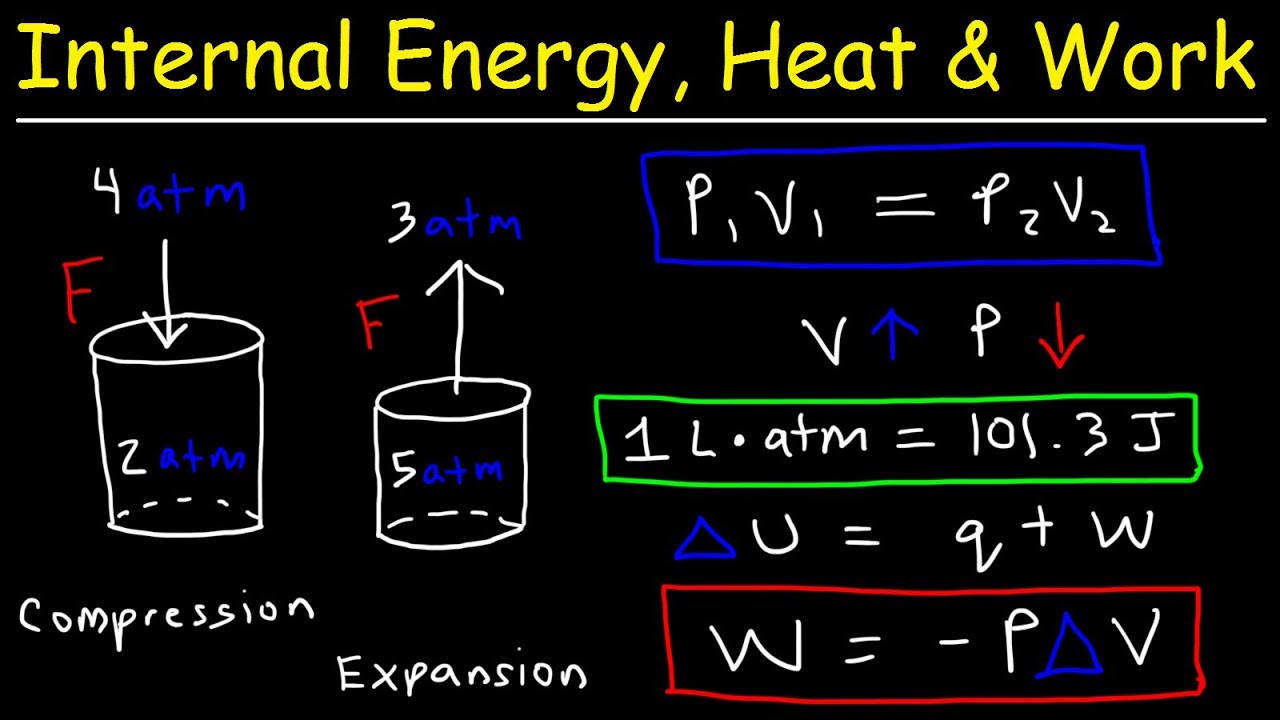
Internal energy, heat, and work thermodynamics, pressure & volume
A "1.32 l" volume of gas has a pressure of "1.00 atm". what will the .
.


(PDF) The pressure-volume-temperature equation of state of MgO derived

Aim: How does changing pressure and temperature affect a gas? - ppt

PPT - Collisions of Gas Particles PowerPoint Presentation, free

Relationships among Pressure, Temperature, Volume, and Amount

Pressure, Volume and Temperature Relationships - Chemistry Tutorial

gases.htm

Critical Constants - Temperature, Pressure, Volume of Real Gas

A 12.0 L gas cylinder has been filled with 5.40 moles of gas. You